The aromatic hydrocarbon benzene, chemical formula C6H6, is the single most-often discussed of the aromatic hydrocarbons. It possesses the quintessential aromatic structure. How is that?
It possesses the Hückel Rule correct number of 4n + 2 number π-electrons, for n = 1, and is flat. In addition, it meets all the other requirements for aromatic compounds. As an aromatic compound, its chemistry is considerably different than it would be if it existed as a mere cyclohexatriene isomer. We will discuss those differences in this, the third installment of our three-part article.
Behavior of Hypothetical Cyclohexatriene
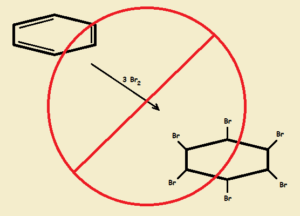
Since cyclohexatriene would behave as if it were merely three conjugated1 double bonds, we would expect the compound to behave as any other alkene—rich in available electrons—only more so. Electron-poor reactant should attack triene at the double bonds, attaching itself through the process called “addition.”
Thus if the deeply red-brown halogen, bromine, is added to the hypothetical triene gradually, the color should disappear until all the double bonds are gone. The reaction result would be hexabromocyclohexane. See Fig. 1
Behavior of the Actuality—Benzene
What happens in reality—when bromine liquid is placed in a reaction vessel with bromine liquid? Since benzene is stabilized through aromaticity—under ordinary circumstances—absolutely nothing happens. If the reaction is forced, under the influence of strong light or a special catalyst, the result is a substitution, rather than an addition, reaction. The product is C6H5Br or bromobenzene. See Figure 2.
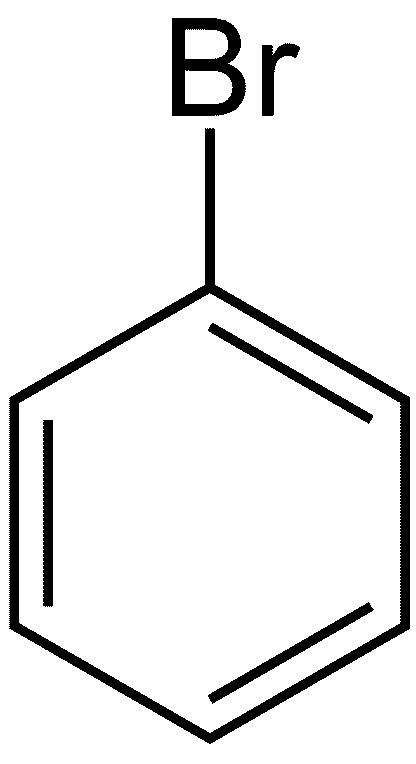
This is because bromine (Br2) seeks electrons, and benzene holds a tighter grip on its electrons, compared to cyclohexatriene. Instead of addition, a hydrogen atom is extracted from the benzene by one of the bromine atoms, producing a molecule of hydrogen bromide (HBr). The other bromine atom attaches at the vacancy point resulting from hydrogen’s departure.
Generalization
In general, then, non-aromatic cyclic hydrocarbons containing double bonds undergo electrophilic2 addition reactions, whereas aromatic cyclic hydrocarbons undergo nucleophilic3 substitution reactions.
It ought to be mentioned that if there is a double-bond-containing, non-aromatic attachment to an aromatic ring, that attachment is capable of undergoing addition reactions. It comes under no protection from the overall molecule’s aromatic portion.
1 Conjugated means strictly alternating double bonds, for instance, -C=C-C=C-C=C-.
2 Electrophilic means, literally, electron-loving.
3 Nucleophilic means electron donating, or the ability to react at an electron-poor site.
Note: You might also enjoy The Persistent Triphenylmethyl Free Radical
References:
- California State U – Dominguez Hills: enzene Structure and the Aromatic Ring
- University of Memphis: Nucleophiles, Electrophiles, and Leaving Groups
← Back to Classic Science
← Home
