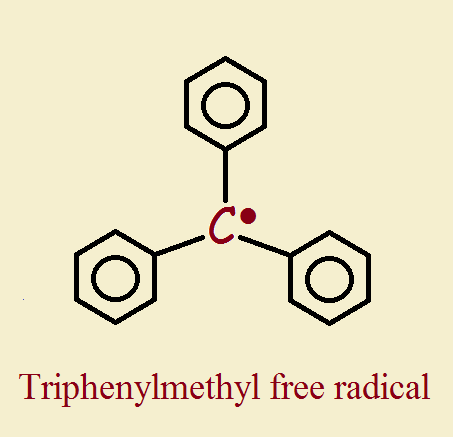
The persistent triphenylmethyl free radical. What is it? What is a free radical and how, and in what sense, is this one “persistent”?
The term free radical is a household word, due to its association with food and with body health. Although the expression is bandied about, the public at large does not know what it means. It relates to the concept of atoms, molecules, and electrons.
A chemical free radical is defined as a molecule having no net electrical charge, that nevertheless, has at least one unpaired valence electron. Do you find this definition hard to follow? Continue reading and view the hyperlinked graphic model, cited in the following paragraph.
Triphenylmethyl Free Radical
The first example of an organic species was identified in 1900 by Moses Gomberg. Here is a computer mouse movable 3D model of the triphenylmethyl radical. This particular radical was particularly easy to study because it is persistent. It is sufficiently stable so that it has an unusually long life expectancy.
The unpaired electron is hampered in reactivity due to sterics (crowding) imposed by the surrounding pendant phenyl groups (C₆H₅‒). This means other species have difficulty reaching and reacting with the triphenylmethyl free radical. Looked at from another perspective, this means this free radical is “protected”. This makes it exceptionally well-suited for scientific study.
Note: You might also enjoy Hückel’s Smallest: The Aromatic Cyclopropenyl Cation

I have never heard of that before. Free radicals – yes – but not “protected” electrons. Never knew there was such a thing!