Sometimes a chemistry or physics concept is misunderstood even by the reader of above-average intelligence and education.
Such is the case with the chemist’s use of the term aromatic resonance.
There are a small number of recognized rules for aromatic behavior. These include:
- cyclic geometery
- planarity
- 4n + 2 contributing, de-localized, conjugated (π) double bonds
An Example of What Aromatic Resonance Is Not
The best and oldest known example is benzene, C₆H₆. Benzene is cyclic (one ring), planar (flat), and has 6 delocalized, conjugated double bonds. Frequently, the organic chemist simply renders benzene’s structure…
There is a carbon atom at each vertex of the hexagonal ring. Attached to each carbon and pointing outward from it is one hydrogen atom.
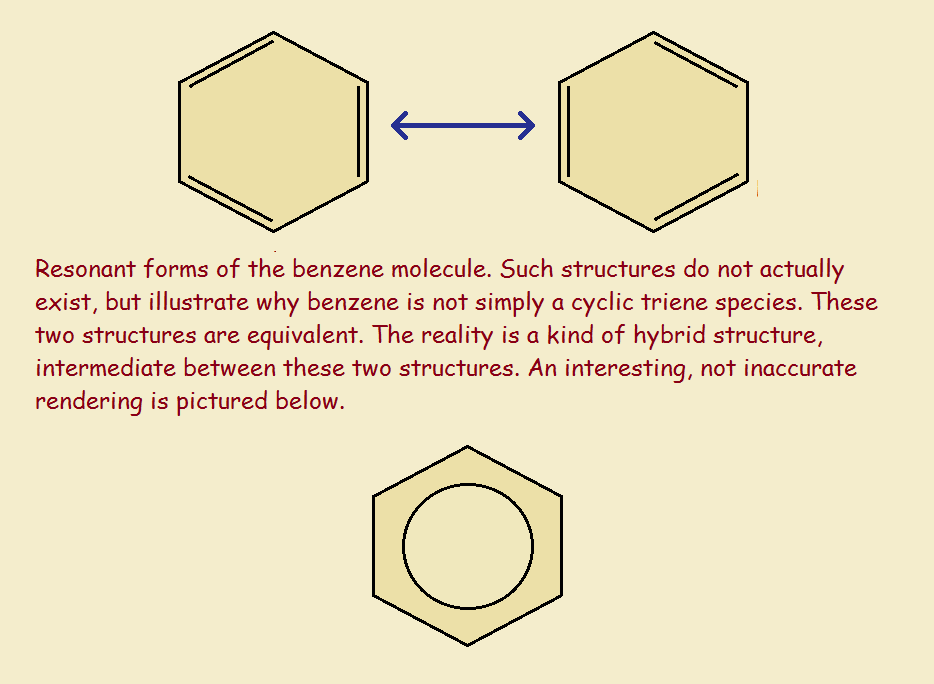
Now each carbon atom has a valence of 4. That is, each carbon forms four bonds. The resultant molecule may be drawn in two equivalent ways.
That these are equivalent should be obvious. Imagine flipping one of the forms over. Since benzene is flat, what does that result in but the second form? But if they are equivalent, does benzene actually form a 50-50 mixture of two equivalent species? And if so, do the two exist in equilibrium?
NOT in Equilibrium
The answer is a resounding No! Benzene does not consist of two or more equal (in equilibrium) forms. It exists as one somewhat intermediate form that does not display two bond varieties, single and double. Each and every bond is intermediate—giving rise to the circle-within-a-hexagon drawing.
Each bond is of equal strength and equal length, approximately equivalent to a 1-1/2 bond, rather than either a single or a double bond. Each has identical energy. Another difference is that the total bond energy is less than what it would have been with alternating single and double bonds.
This energy savings is termed resonance energy. Benzene molecules are more stable (possess less energy) than either resonant form. This increased stability causes the molecule to “want” to engage in substitution reactions, rather than give up the stabilization through addition reactions.
In Summary
To summarize, aromatic resonant forms are only mental constructs that do not actually exist. They merely serve as helpful tools to the organic chemist. These tools help visualize what goes on at the molecular level. Aromatic resonance is not a flip-flop of bonds. Aromatic bonding is stable. It is unmoving.
Note: You might also enjoy Hückel’s Smallest: The Aromatic Cyclopropenyl Cation
References:

If the resonant form has less energy than either of the other two forms, does that imply a loss of energy somewhere when benzene is formed?
Not at all. Rather, on paper, energy is given off.
Sorry, I was unclear, that is what I meant, that the production of benzene is (exothermic?), ie, the benzene is produced in some sort of reaction that creates heat, because the benzene is at a lower energy level than it would be if the two forms co-existed?
If one of the two forms were chemically generated, it would quickly/simultaneously lose energy (exothermic) as it turned to benzene.