 An empirical formula lists the elements of a compound but not the structure. Oxalic acid has the formula C2H2O4. Its structure is HOOC–COOH. Multiple compounds can have the same empirical formula and a different structure.
An empirical formula lists the elements of a compound but not the structure. Oxalic acid has the formula C2H2O4. Its structure is HOOC–COOH. Multiple compounds can have the same empirical formula and a different structure.
The Same Yet Different
Location of a specific kind of bond may make the difference. There are compounds with the same empirical formula in which spatial orientation is the only difference. Sometimes one structure can be changed into another structure of the same formula. Most often compounds having the same formula but different structures are completely unrelated.
Simple Bond Shift
In some cases, a simple bond shift produces different structures with no formula change. An example is 1-butene and 2-butene. The first has the structure H2C=CH-CH2-CH3. The second has the structure H3C-CH=CH-CH3. Both have the empirical formula C4H8. In fact, 2-butene exists in two forms, trans-2-butene and cis-2-butene. All three compounds are similar, but not are the same. For example, each has a unique boiling point,
1-butene –6.4 Degrees C.
Trans-2-butene +1.0 Degrees C.
Cis-2-butene +3.6 Degrees C.
Other properties vary, too.
Spatial Orientation
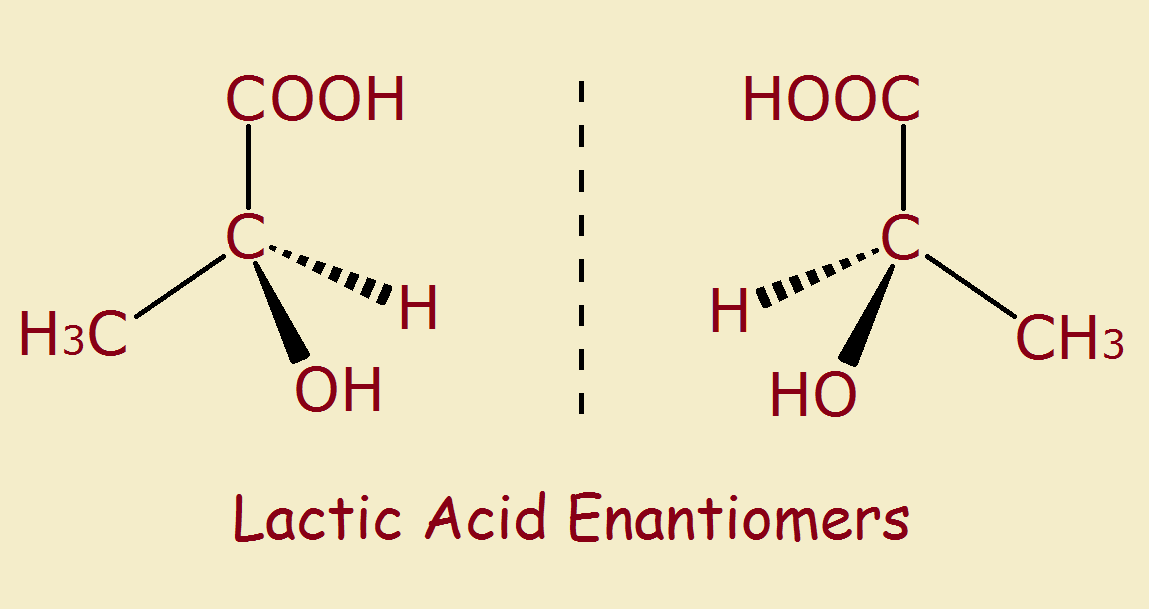
Most compounds have 3D structure. This is because carbon atoms usually bond to four other atoms. If one of those bonds is to another carbon atom, the remaining three bonds may connect to entirely different atoms. This allows for two different compounds, each a mirror image of the other. Mirror image compounds are called enantiomers.
A simple example is 1-chloro-1-bromo-1-fluoroethane. Its formula is C2H3ClBrF. It is easiest when simply written H3C-C(ClBrF).
If the three atoms on the right hand carbon atom are in order chlorine, bromine and iodine, then its mirror image orders them iodine, bromine and chlorine.
Bond-Shift Variants
Consider two compounds of formula C3H6O. Acetaldehyde is written CH3-CH=O. The double bond may shift to produce CH2=CH–OH, ethenol. This reversible bonding is called tautomerism. The concentration of one form over the other depends upon certain factors, such as pH.
As another example, the linkage –C–NH–C=O can tautomerize into –C–N=C–OH. The same is true here. Relative concentration of one form over the other depends on environment.
Same Empirical Formula – Unrelated Structures
Unrelated structures may have the same formula. C4H8 can refer to the ring structure, cyclobutane. It is also the formula for 1-butene, CH2=CH–CH2–CH3.
Or consider allene and propyne. Allene is H2C=C=CH2. Propyne is HC≡C–CH3. Both have the empirical formula C3H4.
Enjoy the video below. It was produced by the Khan Academy. It is titled, Emipirical, molecular, and structural formulas.
Note: You might also enjoy Introduction to Chemistry Subscripts and Superscripts
← Back to Classic Science
← Home
