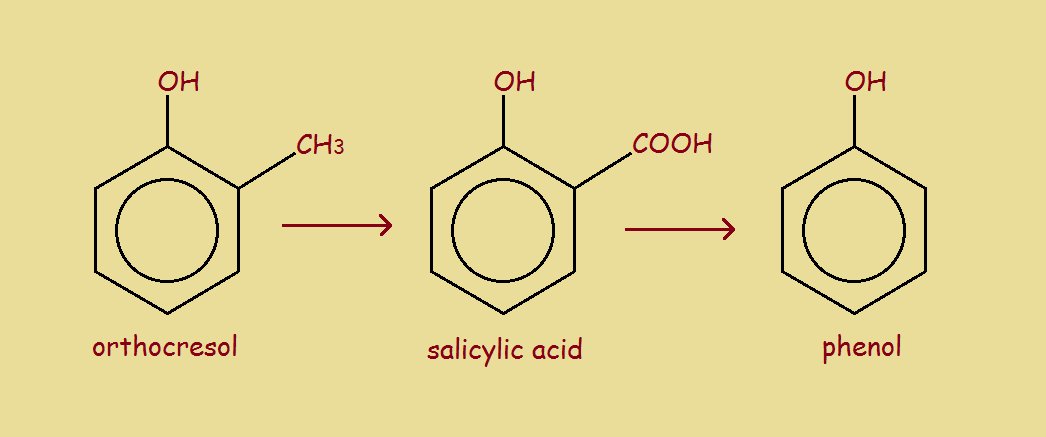
 For one reason or another, it may be desirable to remove a carboxylic acid group (–COOH) from a molecule. This process is called, logically, decarboxylation. It may be the carboxylic group was previously introduced to guide some of steps in a synthesis. What?
For one reason or another, it may be desirable to remove a carboxylic acid group (–COOH) from a molecule. This process is called, logically, decarboxylation. It may be the carboxylic group was previously introduced to guide some of steps in a synthesis. What?
A Simple Example
Consider the simple hexagonal ring, aromatic compound benzene, C6H6. From the top and clockwise, let’s number the carbon atoms, one through six. If we wish to attach a group synthetically to the ring, how do we determine the ring carbon to which it will attach?
It’s simple. They’re all identical. Whichever carbon atom it does attach to becomes the number one carbon atom! But say we want to attach another group to the No. 2 carbon atom. Well, in this situation, the carbon atoms are not identical. The symmetry was broken thanks to the group on the No.1 carbon.
We need to give names to disubstituted benzene that reflects this. We call 1,2-disubstitution ortho, 1,3-disubstitution meta, and 1,4-disubstitution para.
Ortho, Meta and Para
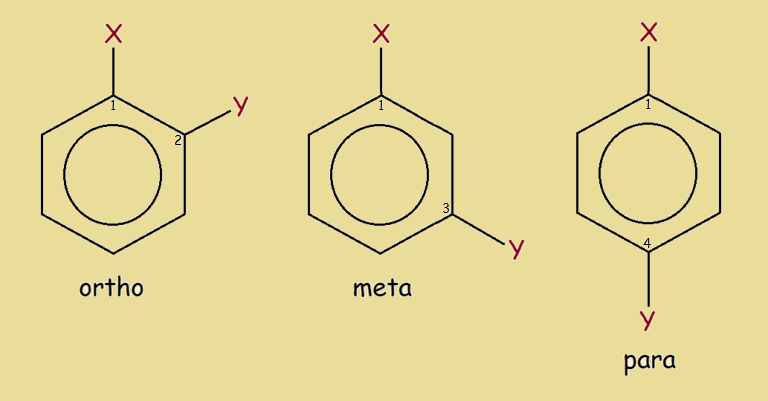
If the substitutions are identical, we refer to ortho-, meta- and para- disubstituted benzene as isomers. Unfortunately (or fortunately, depending upon how you look at it), these isomers may not be straightforward in their preparation. This is because of electronic considerations, which we will not discuss here.
Suffice it to say: we have meta-directing groups and ortho-para-directing groups. Here’s what we mean by that. When a benzene ring has a first substituent group already in place, that group exerts a controlling influence over where a second group can go.
Ortho-para-directors include (among others) alkyl groups, phenyl groups and amino groups.
Meta-directors include (again, among others) nitro groups, nitriles and carbonyl containing groups such as aldehydes, ketones, carboxylic acids and esters.
Turn Apples Into Oranges
So say you have toluene (C6H5CH3) as your starting material. Methyl is an alkyl group and, hence, an ortho-para-director. But you want nitro groups at the 3 and 5 positions. And after that, you want to dump the methyl group!
You could turn apples into oranges. That is, you could convert (through oxidation) the methyl group into a carboxylic acid group, a meta-director. Through oxidation, you do so. You now have benzoic acid, C6H5COOH. You add the two nitro groups.
But you didn’t want the methyl group nor its replacement, the carboxylic acid group. That is, you wish to carry out decarboxylation to yield the product now numbered 1,3-dinitrobenzene.
Decarboxylation
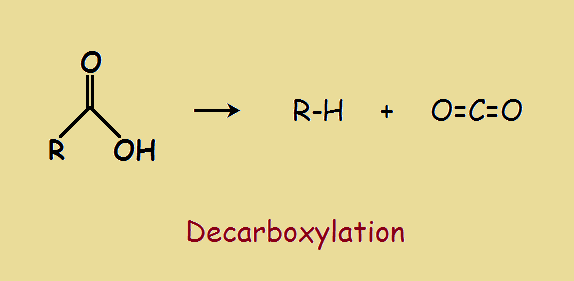
The actual decarboxylation process is relatively simple. Heating in the presence of a catalyst releases carbon dioxide, CO2 from the group, as illustrated in the image. What it boils down to is, you wish to perform chemical grafting and amputation… This is the heart of organic synthesis, the molding of the old to gain access to the new.
Note: You might also enjoy Keto Enol Tautomerism
References:
- UCLA Chemistry: Illustrated Glossary of Organic Chemistry: Ortho-Para director
- UCLA Chemistry: Illustrated Glossary of Organic Chemistry: Meta director
