 Carbon readily bonds to itself. This unusual characteristic allows the existence of a whole host of hydrocarbons, some of which assume amazing geometric shapes.
Carbon readily bonds to itself. This unusual characteristic allows the existence of a whole host of hydrocarbons, some of which assume amazing geometric shapes.
Cyclopropane (C3H6) looks like an equilateral triangle.
Cubane (C8H4) is a cube. In recent years, Bucky balls, graphene sheets and nanotubes have become household topics.
Diamondoids
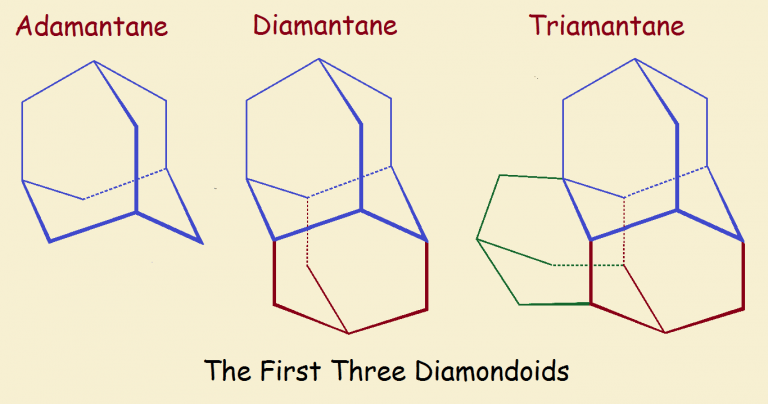
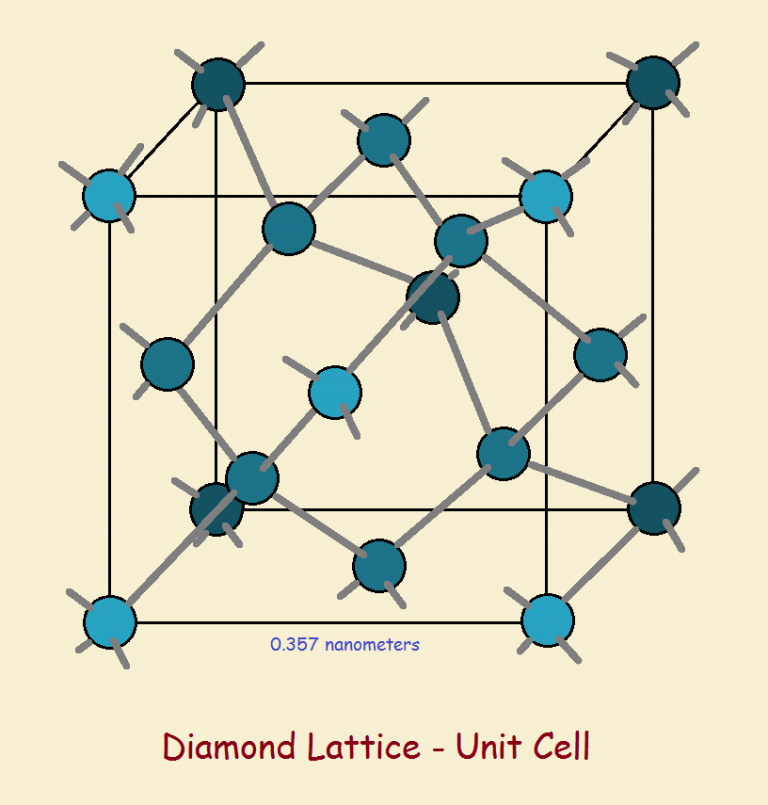
Diamondoids are compounds of hydrogen and carbon consisting of cage-like structures that resemble the diamond lattice.
Carbon-carbon bond lengths are 1.54 Ångstrom units (1 Å = 10-8 centimeters) in length both for diamond and for diamantane. But the structures themselves are difficult to compare.
Please take some time to ponder the unit cell image for diamond and the chemical structures of the first three diamondoids of the series, adamantane (C10H16), diamantane (C14H20), and triamantane (C18H24).
Diamondoid to Diamond?
Diamond is pure carbon. Diamondoids such as adamantane are carbon plus hydrogen. Hydrogen atoms are the smallest of the atoms, and can readily escape most crystal lattices. So it would seem logical to try making diamond from diamondoids. In fact, the very thing has been accomplished using adamantane and shock waves.
But what suggests the conversion of a cage-like hydrocarbon into a diamond can occur? Well, realistically, not a diamond, but large numbers of very tiny diamonds, nanodiamonds?
Diamond particles have been discovered in meteorites and other impact events, including explosions. If they can come about unintentionally, they can be produced intentionally, using the correct parameters…for example, shock waves.
Of What Use Is It?
Research on the conversion of diamondoids to diamond nanoparticles is still in its infancy, but anticipated results suggest nanodiamonds will be useful in drug delivery, electronics, and quantum mechanical applications. How so?
In Drug Delivery
The Iranian Journal of Biotechnology suggests nanodiamonds would have excellent surface properties and would be unreactive, photostable and non-toxic. They would also assure the correct dosage of medication is delivered to the correct location. To use an example, a medication is needed in “the gut”. The acid environment would not react with the nanodiamonds.
In Electronics
As an example, Vanderbilt University engineers have developed nanodiamond thin-film microelectronic devices that can function in high radiation, low temperature environments. These devices require less power than corresponding silicon-based devices.
Quantum Mechanical Applications
Given the proper growing conditions, nanodiamonds with lattice imperfections can be produced to serve as cages that trap substances within them such as nitrogen gas. Such nanodiamonds can be stimulated to emit intense light. This property has been called superradiance. In conjunction with magnetic fields, it might be used in a wide variety of ways, including mapping aircraft location and visualizing drug delivery sites, such as those mentioned above.
Nanodiamond Research: The Future
The reader may recall the days when Hollywood spoke of the car of the future, the city of the future, and the man of tomorrow. Such fantasies never experienced fruition. But with so many institutions behind such projects as nanodiamond research, it can be strongly suspected something productive will result. Then it may prove to be the case that diamonds are not only a girl’s best friend.
Note: You might also enjoy Pyrophoricity: How Does It Work?
References:
- Imperial College of London: Ring-flipping in cyclohexane in a different light
- Journal of Structural Chemistry Vol. 57, No. 7, pp. 1469-1476, 2016: Investigation of adamantane-diamond transformation. The radical mechanism of the formation of diamond nanoparticles under shock-wave action on adamantane
- Wiley Online Library: Diamonds are a Chemist’s Best Friend: Diamondoid Chemistry Beyond Adamantane – Hartmut Schwertfeger
- Gizmodo: How These Microscopic Diamonds Are Going to Shape the Future
- Drexel Nanomaterials Group: Nanodiamonds for Drug Delivery Applications
- Sustainable Nano: Nuclear Proliferation & Sustainability: the History of Nanodiamonds
- National Institutes of Health: Role of Nanodiamonds in Drug Delivery and Stem Cell Therapy
- Photonics Media: Nanodiamonds are a circuit’s best friend
- Phys.org: Tiny diamonds light the way for new quantum technologies
