Although words can have multiple meanings, we will consider only the difference between an orbit and an orbital, the path of planets and that of electrons in atoms. Planets (and other astronomy objects) travel in orbits. Jupiter and Haley’s Comet orbit the Sun. Electrostatic forces and selection rules hold electrons in specific atomic orbitals.
What is an Orbit?
Orbits are generally elliptical in shape. Ellipses are circles that are stretched out along one axis. The degree of stretching is termed eccentricity. The greater the stretch, the greater the eccentricity. Consider the figure below for comparison.
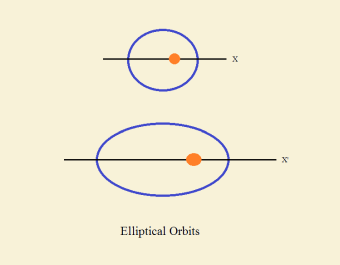
The object doing the orbiting travels strictly in the path of the orbit. It does not deviate. In this respect, orbits of astronomical objects are different from atomic orbitals. At the sub-atomic level, quantum physics takes precedence.
Atomic Orbitals
Although the orbit of an astronomical object can be illustrated simply by drawing an ellipse, in reality no planar mathematical representation suffices for the atomic orbital of an electron. In fact, even drawing a 3D curve does not truly suffice. To illustrate, consider the orbital of a single electron in an atom of hydrogen.
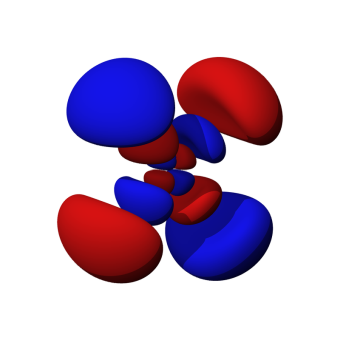
The shape of the orbital is spherical. Does this mean, then, that the electron of a hydrogen atom is restricted to traveling anywhere along the outer surface of the orbital sphere? No, it does not. In fact, a closer approximation would be that the electron could travel anywhere inside that sphere. But even that approximation is not correct.
Honing In
A more accurate statement would be that the electron travels within that sphere a particular percentage of the time—say 90%. There is almost nowhere in space an atomic electron could not be. One might expect this on the basis of the Heisenberg uncertainty principle.

An astute mental picture might be to think of an electron as a highly energetic particle that wants to roam free, though it is restrained from doing so entirely by house rules. Or try a child, told he can play outside, but must not leave the property. An electron does not merely rotate in circles about a set path, as an astronomical object does. A higher authority dictates what it can and cannot do.
Quantum Rules – Quantum Numbers
Quantum rules, quantum numbers—four of them—dictate the shape and size of any particular orbital about a particular atom. In fact, very few atomic orbitals are spherical. They assume a variety of shapes, as the image associated with this article demonstrates.
Note: You might also enjoy Cesium Auride Has Relativistic Electrons?
References:

We would all be in trouble if planets had orbitals instead of travelling in orbits then!
The dark matter that lies in between objects might be similar to the forces that lie in between atoms. But on a different energy scale. I think this was a very good example of the differences between orbits and orbitals.
Physicists have found astronomical objects and atomic objects do not correlate in behavior. Thanks for reading, Anthony.