Energy (E) is crucial for life; there is no life apart from energy. Fortunately energy derived from matter exists abundantly. It is readily obtainable.
Chemical E
Our bodies require small installments of energy. This leads us to the dinner table. Our body breaks down the food we eat, liberating chemical energy that keeps us functioning and warm. For instance, there is the digestion of starch into sugar and energy. Sugar is further broken down into carbon dioxide, water, and still more energy.
This form of energy can also be called molecular energy, since it comes from the breaking down of molecules. This involves the breaking of chemical bonds between some of the atoms of a molecule. All the atoms remain the same, though. They are unchanged.
Atomic E
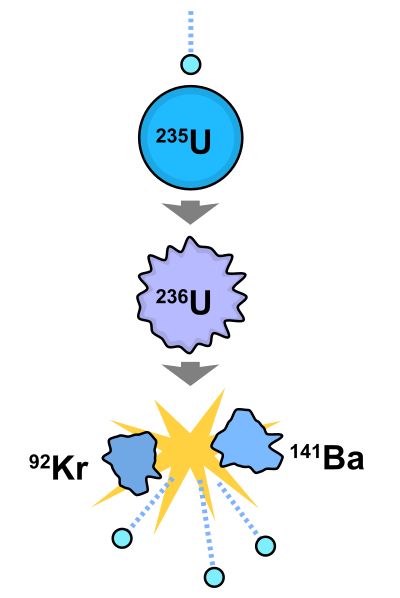
Chemical energy sustains our bodies, but it by no means represents all the available energy we can obtain from matter. Chemical energy alone constitutes only a tiny, tiny fraction of the total available. For instance a modest quantity of Uranium-235 enables the deadly force of an atom bomb. Even that level of energy is small compared to the total energy contained in atoms.
The uranium is not destroyed—its atoms are merely split into smaller atoms. These smaller atoms still contain the bulk of the energy. At least in theory, these atoms can be made to give up all their energy. In the example image, the tiny blue particles represent neutrons that initiate the splitting reaction.
E from Annihilation
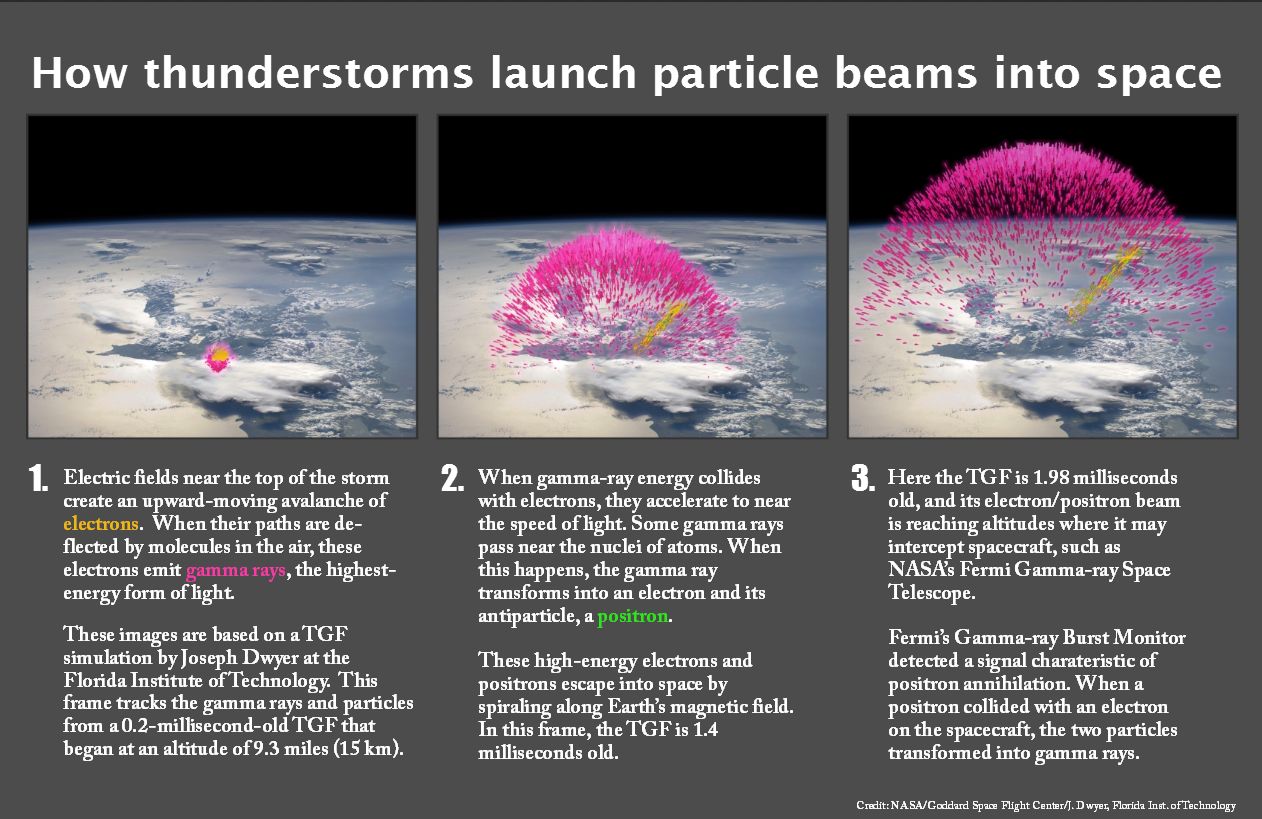
Einstein’s well-known equation, E = mc² informs us that matter and energy are interchangeable. Thus there should be a way of converting atoms completely, 100%, into pure energy. The question is, how? It can be accomplished by combining matter with antimatter. What is antimatter?
Antimatter is a form of matter consisting of antiparticles such as antiprotons, antineutrons, and positrons that, when combined with an equal quantity of ordinary matter, is completely annihilated, in accord with Einstein’s equation. Conversely, as the equal sign in Einstein’s equation suggests, energy can be converted into equal quantities of matter and antimatter. This is sometimes observed in thunderstorms.
Note: You might also enjoy What are Hybrid Atomic Orbitals?
References:
← Back to Quirky Science Nuggets
← Home

Interesting article.
E = mc² – it’s like being a kid in a candy store: all those goodies behind the glass and out of reach! We know how E = mc² works and can see it in action, but we aren’t smart enough – yet – to apply it in any useful way to power our energy-hungry world.