
An atom consists of two components – a nucleus and its orbiting electrons. Nuclei contain neutrons and protons bound together by nuclear force. Electrons travel in well-defined atomic orbitals outside the nucleus. Orbitals come in different shapes. They contain up to two electrons each. A collection of orbits forms an electron shell. Atoms can have more than one shell.
Orbitals and shells are identified by letters and numbers. The details are beyond the scope of this article; however, atoms begin filling electron orbitals in the order,
Orbitals: s, p, d, f…
Shells: 1, 2, 3, 4…
First Elements
Thus the first ten elements fill their orbitals and shells,
Hydrogen 1s¹
Helium 1s²
Lithium 1s² 2s¹
Beryllium 1s² 2s²
Boron 1s² 2s² 2p¹
Carbon 1s² 2s² 2p²
Nitrogen 1s² 2s² 2p³
Oxygen 1s² 2s² 2p⁴
Fluorine 1s² 2s² 2p⁵
Neon 1s² 2s² 2p⁶
To illustrate, neon has two s electrons in its first shell. It has six p electrons in its second shell.
Methane
We will begin with methane, a common constituent of “swamp gas,” molecular formula CH4. This molecule has four atoms – one carbon atom and four hydrogen atoms – and so it has to form four carbon-to-hydrogen bonds. Each bond requires two electrons.
Hydrogen, as we see from the above list, has only one 1s electron available. Since there are four hydrogen atoms in methane, hydrogen donates a total of four electrons to the single carbon atom involved. The carbon atom, receiving these four electrons takes on a new electron configuration,
1s² 2s² 2p⁶
The different orbitals, s, p, d, f, and so forth, are shaped considerably different from each other. All s orbitals are spherical, while p orbitals are cylindrical.
Since each bond requires two electrons, it appears three of the bonds would use the six p electrons and one of them would use the two s electrons. If that was what actually happened, the bonds would be different; but, it is not the case.
Formation of Hybrid Atomic Orbitals
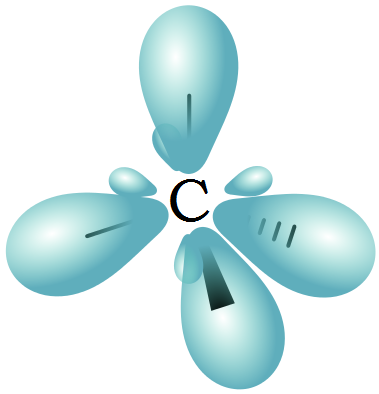
Since all the carbon-hydrogen bonds are the same, the electrons must all be equivalent. Yet some of them are s electrons and some of them are p electrons. Something special has to happen, and it does – hybridization.
If all eight of shell 2’s electrons are put in a pool in common, we can then draw from that pool. Since two different kinds of orbital electrons are used – two electrons from the s orbital and six electrons from the p orbital – we will call the resulting molecular orbitals sp³ orbitals. The eight sp³ electrons form four bonds. Methane actually is a carbon atom surrounded by four hydrogen atoms arranged tetrahedrally.
Discussion
There are other types of orbital hybridization – the combining of different orbitals whether s, p, d, f, or some other – into a collection of new orbitals each the same. Evidence that a suggested hybridization is true lies in its actually being demonstrated in nature.
Thus, what might seem to be an artificial construct is actually what occurs. Such theories given a solid basis help scientists to learn more about the matter in the world around us.
Note: You might also enjoy What is a Hydronium Ion?
← Back to Classic Science
← Home
