 Chemical explosives are unstable¹ solids (occasionally liquids) that decompose rapidly, releasing large quantities of gas. The sudden volume increase pushes violently against whatever contains the explosive, reducing it to shrapnel and releasing a thunderous noise.
Chemical explosives are unstable¹ solids (occasionally liquids) that decompose rapidly, releasing large quantities of gas. The sudden volume increase pushes violently against whatever contains the explosive, reducing it to shrapnel and releasing a thunderous noise.
Many explosives are nitrogen-containing substances, such as trinitrotoluene (TNT), nitroglycerin, and the picrates. Additional historic explosives include the fulminates, and yes, fulminates contain nitrogen as well. The most famous fulminate is mercuric fulminate, officially named mercury(II) fulminate. Its chemical composition is,
Hg(CNO)2
It consists of one mercury atom and two atoms each of carbon, nitrogen, and oxygen. In greater detail, it can be written,
Hg+2(C≡N=O)2
or
Hg+2(C≡N+–O–)2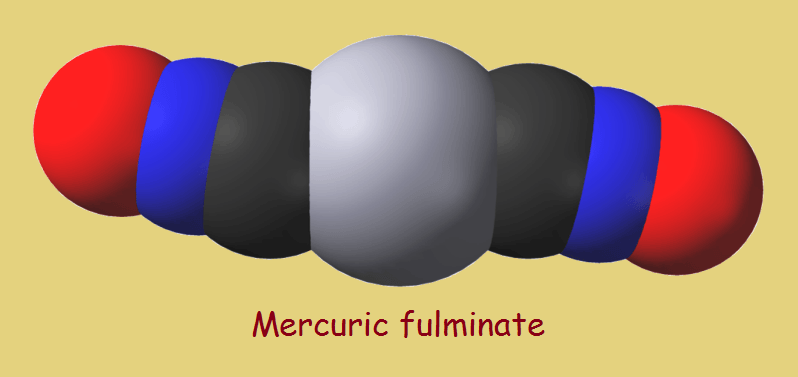
Do Fulminates Remind You of Other Chemicals?
Perhaps the anionic fulminate ion, –CNO– reminds you of the cyanate anion, –OCN– or isocyanate anion, –NCO? And well it should, even though these are considerably different. But don’t they all consist of one nitrogen, one carbon, and one oxygen atom? How different can they be? Well consider the bonding…
Fulminate: –C≡N=O
Cyanate: –O–C≡N
Isocyanate: –N=C=O
The bonding in each case is different, and the atom bonded to, is likewise different. The fulminate exhibits nitrogen at its highest oxidation level.
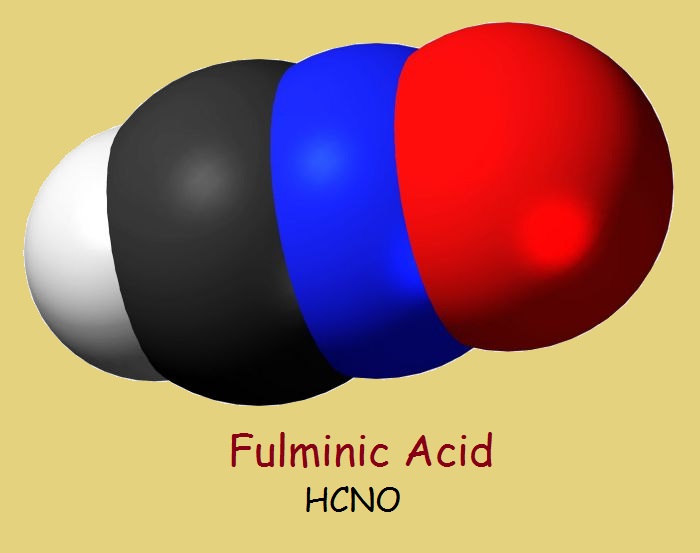
Fulminic Acid
The parent acid compound can be simply written in one of two ways:
H–C+=N–O–
and
H–C≡N+–O–
Once again, IUPAC comes the rescue (not). They’ve named this simple four-atom compound oxidoazaniumylidynemethane. You should be given a prize if you can pronounce that word! Yes, I can say it, but I’ve had years of practice.
Fulminates
The fulminates are considered primary explosives. Primary explosives are explosives that detonate by ignition, impact, or heat of sufficient magnitude. They require no detonator. In fact, unstable fulminates can be considered “touchy”.
To get a feel for just how explosive mercuric fulminate actually is, consider this 3-minute Myth Busters video…
In Conclusion
If you’ve read this article and watched its embedded video, you now realize why fulminates are included among the nitrogenous explosives that need to be utilized with the greatest of care. Does it not seem to be good advice that you, the reader, not “try this at home?”
1 An explosive is not necessarily unstable in every environment, but most frequently (and usefully) under a particular stimulus, such as a physical or electrical shock.
Note: You might also enjoy Chemical Explosives: Picric Acid and Picrates
← Back to Classic Science
← Home

Wow! 5 grams and it blew that pumpkin to shreds! That’s only 1/6th of an ounce. Very dangerous.