Who doesn’t love a ham and cheese sandwich? Or a peanut butter and jelly sandwich? Well, there are special chemical substances that resemble sandwiches.
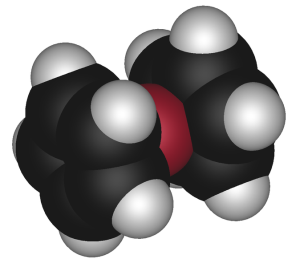
There is, for example, the sandwich compound ferrocene, (C₅H₅)₂Fe. It consists of an atom of iron “sandwiched” between two cyclopentadiene molecules.
Sandwich Compound
The classification of such metallic organic compounds is metallocene. D-orbital electrons in the iron atom add to the pi-electrons of two cyclopentadiene molecules. This stabilizes the structure, since the cyclopentadienyl anion (-1 charge) has aromatic properties. The iron atom adopts, in effect, a ferrous (+2) electrical charge.
Other Metallocenes
In general, metallocenes have mostly been of laboratory interest. Some do exhibit a marked level of importance. One application is in catalysis.
Other metallocenes include manganocene, ruthenocene, titanocene, etc. This well-illustrates how metallocenes are named. Interestingly, not all metallocenes employ transition metals.
A Question by the Author
Even as the iron atom “slab of meat” in ferrocene adds to the stability of the structure because of (in effect) changing the two cyclopentadiene “slices of bread” into something like two aromatic cyclopentadiene structures, could a different atom unite with other ring structures and resemble an aromatic structure?
Note: You might also enjoy The Aromatic Cyclopentadienyl Anion
References:
