Carbon ring strain? What is that? Let’s begin at the beginning.
The element carbon is one of a small number of elements that can bond to itself repeatedly. Compare gases such as oxygen (O2), called diatomic gases because generally only two atoms unite. Oxygen is exceptional in that it does form the triatomic molecule, ozone (O3). However, carbon can form even lengthy chains.
Thus, carbon can form, not only methane (CH4), but ethane (H3C-CH3), propane (H3C-CH2-CH3), butane (H3C-CH2-CH2-CH3), pentane (H3C-CH2-CH2-CH2-CH3), and so on. Curiously, carbon can also close those chains to form rings, much as a woman can close a string of pearls about her neck using a clasp.
Cyclization
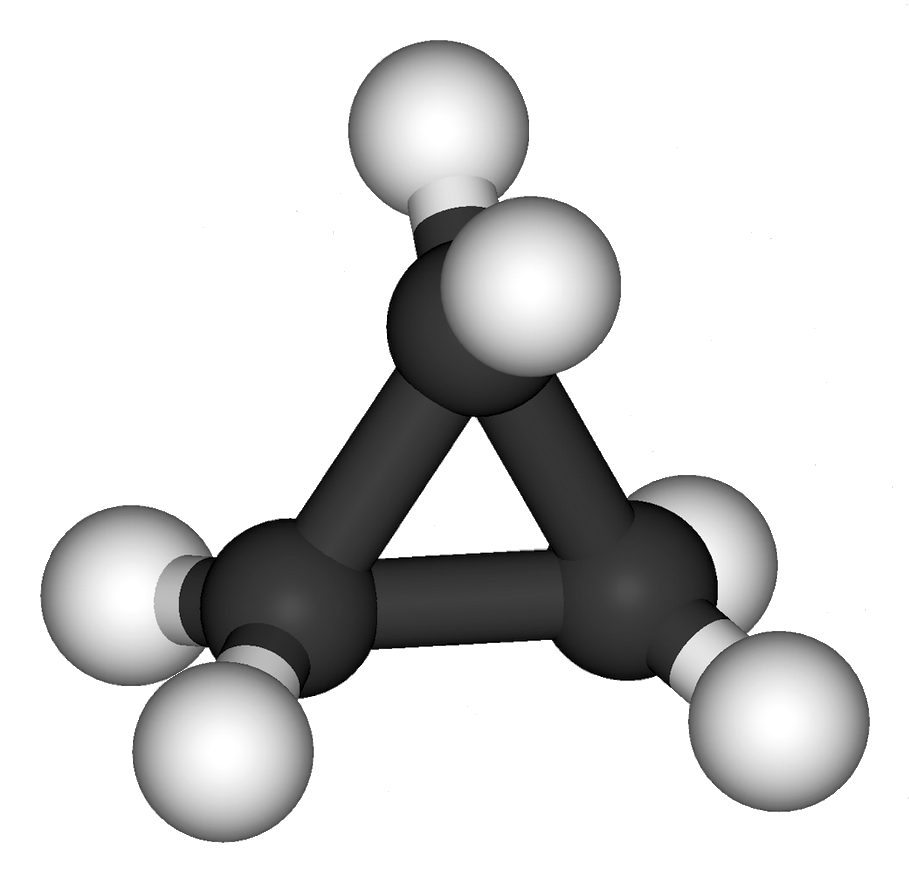
Although methane cannot so close, nor can ethane, the higher analogs can close to form cyclic versions of their linear counterparts. Propane forms cyclopropane with, of course, the elimination of two atoms of hydrogen. The structure of cyclopropane may be seen in the image associated with this article.
Butane becomes cyclobutane, pentane becomes cyclopentane, hexane, heptane, and octane become cyclohexane, cycloheptane, and cyclooctane. Among these cyclic hydrocarbons¹ an interesting property is illustrated – that of ring strain. Ring strain exists because chemical bond varieties seek a particular geometry.
Carbon SP³ Geometry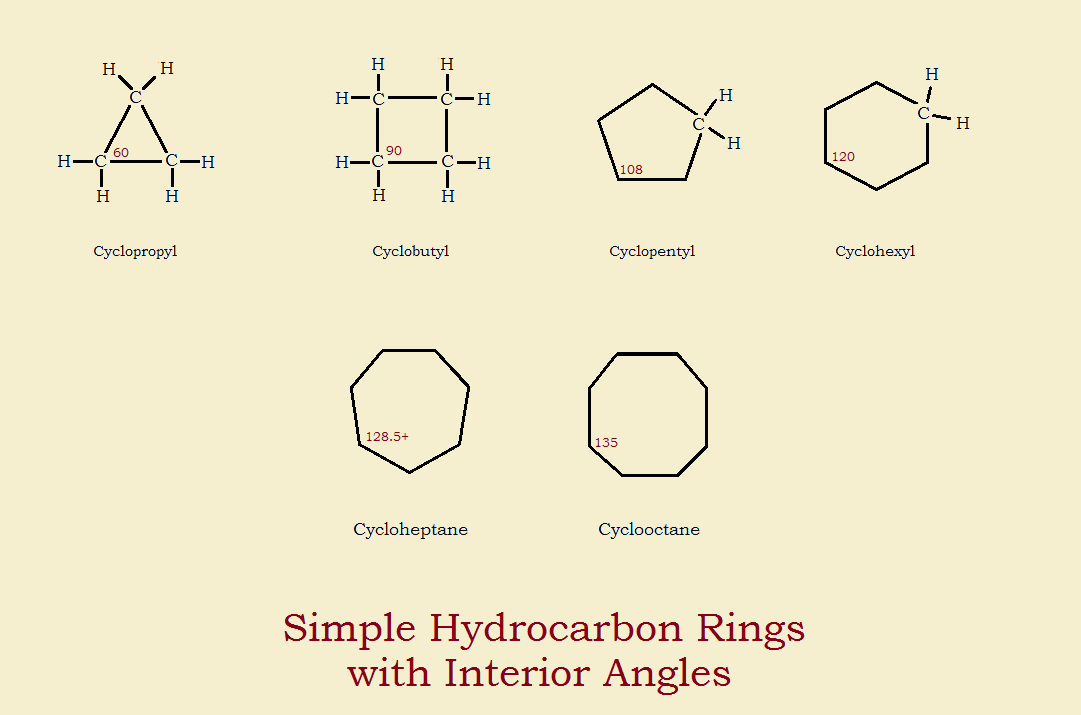
Consider the simplest hydrocarbon, methane (CH4), which possesses the so-called sp³geometry.² The four hydrogen atoms of methane form a tetrahedron about the carbon atom. The angles formed are approximately 109.5°. Now if we consider the cyclopentane molecule spectroscopically, we see the internal angles are each about 108°. This almost exactly conforms to the ideal 109.5°.
As we go up the list, toward cyclooctane, each of the bonds can approach the ideal, as well, by ring puckering. However, such is not the case as we go down the list, to cyclobutane and cyclopropane. Those structures cannot pucker at all, and the angles are less than ideal. Cyclobutane possesses internal angles roughly 90°.³ Cyclopropane has an even worse situation-internal angles of 60°.
Ring Strain
The lack of ideal bond angle causes a decrease in bond strength. The deviation from ideal is called strain. Bonds in cyclobutane, and especially in cyclopropane, are weaker. Hence, these molecules lack stability. They are easily opened by an assortment of chemical and physical processes.
¹ These molecules are termed hydrocarbons because they consist solely of the elements hydrogen and carbon.
² SP³ refers to the theory that says one s and three p atomic orbitals are used to form 4 identical sp³ molecular orbitals.
³ Interestingly, the molecule cyclobutane is not coplanar. The ring is slightly puckered to lessen eclipsing interactions.
Note: You might also enjoy Hückel’s Smallest: The Aromatic Cyclopropenyl Cation
References:

Very informative explanation.