 In your current course of organic chemistry, you’re studying sugars. You notice in a text or see on the web a particular sugar, you’ve searched for by name. What is its true structure? You see it drawn as a chain structure with pendant groups. As you read about it, you see reference to another structure… a cyclic structure! What gives?
In your current course of organic chemistry, you’re studying sugars. You notice in a text or see on the web a particular sugar, you’ve searched for by name. What is its true structure? You see it drawn as a chain structure with pendant groups. As you read about it, you see reference to another structure… a cyclic structure! What gives?
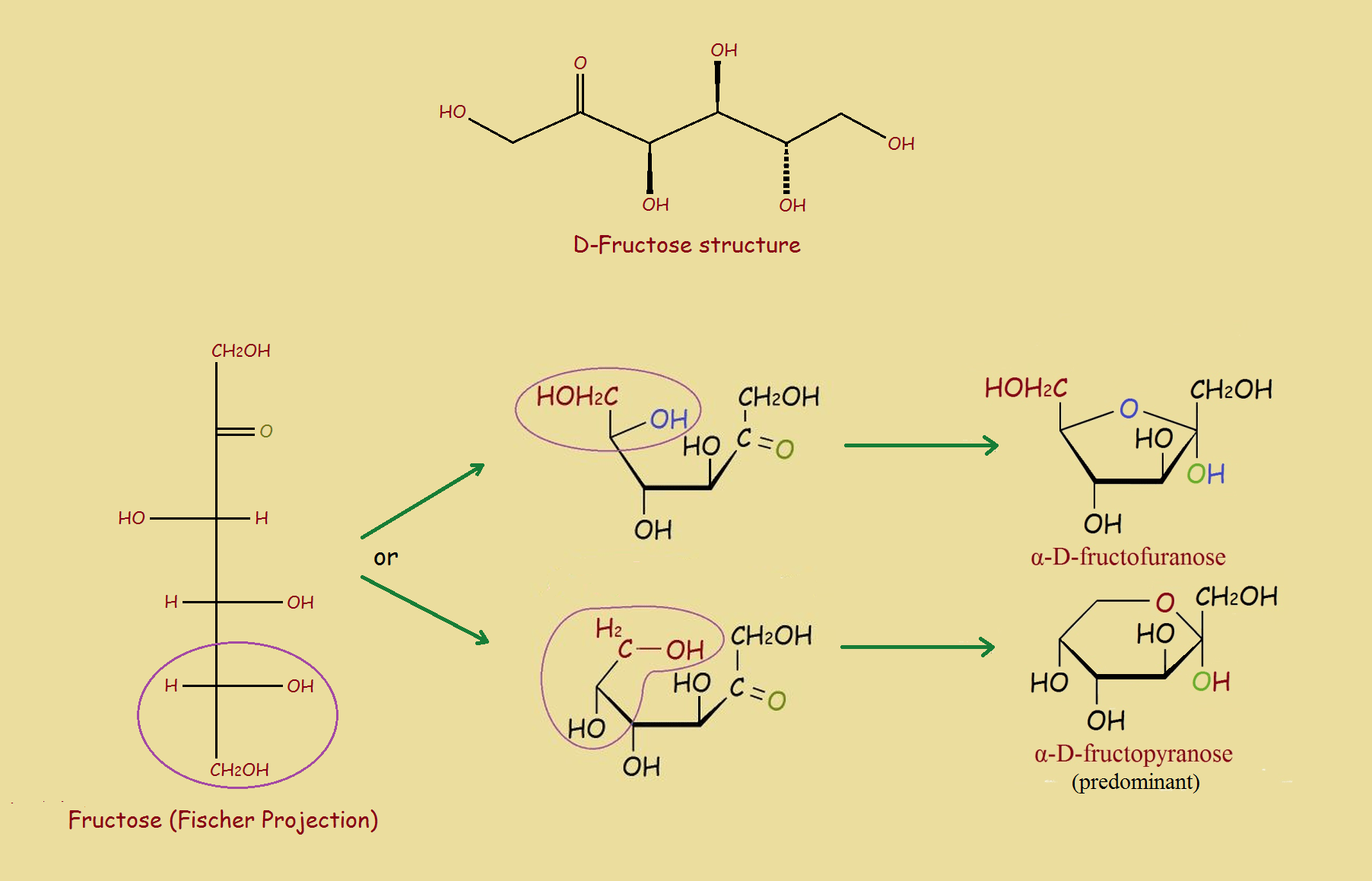
Sugars: Example Fructose
Some chemicals undergo change with the most minimal modification of environment. One example is keto-enol tautomerism.
Fructose and certain other sugars experience something similar. It reacts reversibly, to form two cyclic hemiketals.
Fructose Hemiketals
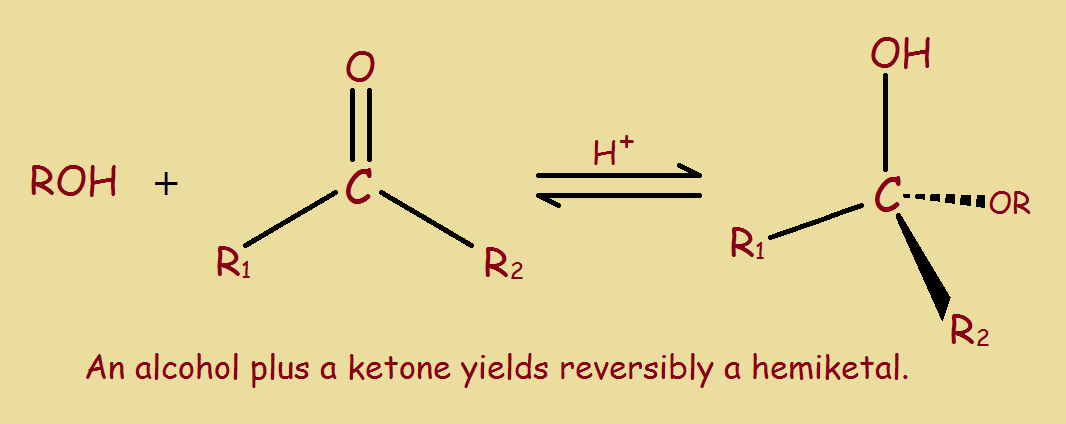
A hemiketal forms by combination of an alcohol group with a ketone group. Fructose supplies both reactive groups, internally. For the generic reaction for hemiketal formation, see the accompanying illustration.
Note the presence of acid favors cyclization. Logically, an alkaline environment would disfavor cyclization.
Fischer Projections
You’ve doubtless noted our featured image includes a Fischer projection. These projections, developed at the end of the 19th century, enable fast comparisons of isomers of a compound. They can also be sketched quickly.
Philosophically Speaking
Some might argue the chain structure and the cyclic structure are two different compounds. However, some of each form coexists with that of the others. In addition, each of the forms is given a separate name, so they can individually be identified, if that is desired.
Note: You might also enjoy Chocolate Alkaloid Theobromine
