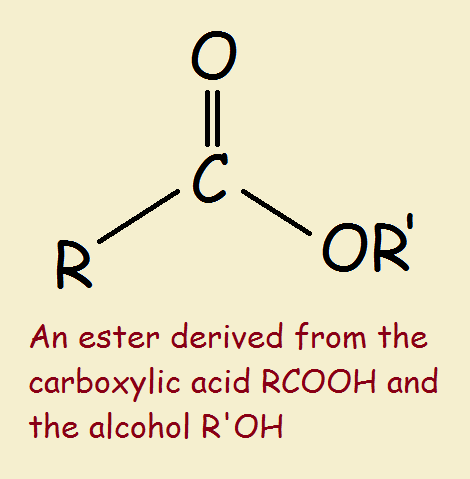
Many esters may be directly prepared by reacting an alcohol with an organic acid.1 To better understand what an ester is, it is important to understand why carbon is so special.
Carbon Bonds to Itself
Frequently, inorganic compounds are relatively small structures. For instance, barium metal, Ba, reacts readily with chlorine gas, Cl2, to give barium chloride, BaCl2.
Ba + Cl2 → BaCl2
Notice barium atoms do not join to each other to form a chain, ring, or some other complex structure. However, carbon – the main element of organic compounds – can and often does bond to itself, forming larger molecules. For instance, diethyl ether is written,
H3C-CH2-O-CH2-CH3
Two of the carbon atoms on the left are bonded to one another, as are the two on the right. In fact, chains and or rings of six, eight, or even a dozen or more carbon atoms are common. Some organic complexes even contain thousands of atoms within a single molecule. One example is deoxyribonucleic acid (DNA).
Alcohols
Alcohols consist of a basic carbon structure with at least one hydroxyl group (-OH group) attached. An hydroxyl group is water with one hydrogen atom removed. Since the shape and structure of an alcohol can vary considerably, it is easiest to symbolize them as R-OH. Some examples of R are,
R = CH3–, C2H5–, C3H7-, etc.
Inorganic Acids and Organic Acids
Hydrochloric acid, or HCl, is an excellent example of an inorganic acid (notice the absence of carbon). The hydrogen readily departs from the chlorine atom, leaving its electron behind. Readiness of departure means HCl is a strong, rather than a weak acid.
Organic acids may be written RCOOH. Again, the hydrogen is mobile, though most often, the acid is weaker than an inorganic acid.
RCOOH → RCOO– + H+
Formation of Esters
Below is the reaction for the formation of methyl propanoate, by the combination of methyl alcohol, CH3-OH and propanoic acid, CH3-CH2-COOH.
CH3-OH + HOOC-CH2-CH3 → CH3-OOC-CH2-CH3 + H2O
This tells us that the hydroxyl of the alcohol combines with hydrogen of the acid2 to form water, while the remaining two organic (carbon-containing) segments unite to form a molecule of ester (rewritten as CH3CH2-COO-CH3).
As can be seen from the above, the general formula for a simple ester is R-COOR’, where the prime on the second R merely means it that R may be different from the first R.
Concluding Remarks
During the twentieth century, molecules were first constructed containing many ester linkages in single molecules. Such molecules are called polyesters. They are used in the manufacture of textiles, including clothing.
1 It should be noted that there are various methods of forming esters, but the simplest way of visualizing is by means of the “alcohol + organic acid” method. Also it should be noted that occasionally a compound is called ester even if an inorganic acid is used, such as a phosphoric acid “ester.”
2 Actually, the hydroxyl of the alcohol may combine with hydrogen from sulfuric acid added for that purpose. Sulfuric acid also ties up the water formed during the reaction so it doesn’t interfere with reaction completion.
Note: You might also enjoy Decarboxylation of Carboxylic Acids Useful in Synthesis
References:
