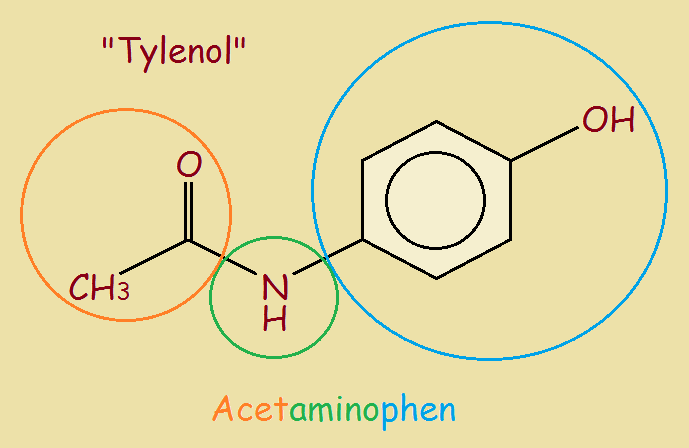
 Tylenol® is a trade name for acetaminophen.
Tylenol® is a trade name for acetaminophen.
What’s in a Name?
Does acetyl remind you of acetic acid or vinegar? Acetic acid is CH3COOH. The acetyl group is written CH3CO–.
Amino is familiar too, as in amino acid? The basic amino group is written NH2–, though at times one or both of the hydrogen atoms are replaced by something else, as it is for Tylenol.
Finally, “phen” stands for phenyl. Remove one atom from a benzene ring and you have phenyl. C₆H₅– is the simplest phenyl ring. Again, hydrogen atoms may be replaced with something else.
The end result of putting these together to form acetaminophen is seen in the included image. That was simple, no? Well then, what about the more official name, N-acetyl-para-aminophenol?
The part that reads N-acetyl tells us that the acetyl group, CH3CO–, is attached to the nitrogen atom rather than to a carbon atom. That’s a no-brainer, right? What about the phenol part? Notice it says phenol, not phenyl. Why is that?
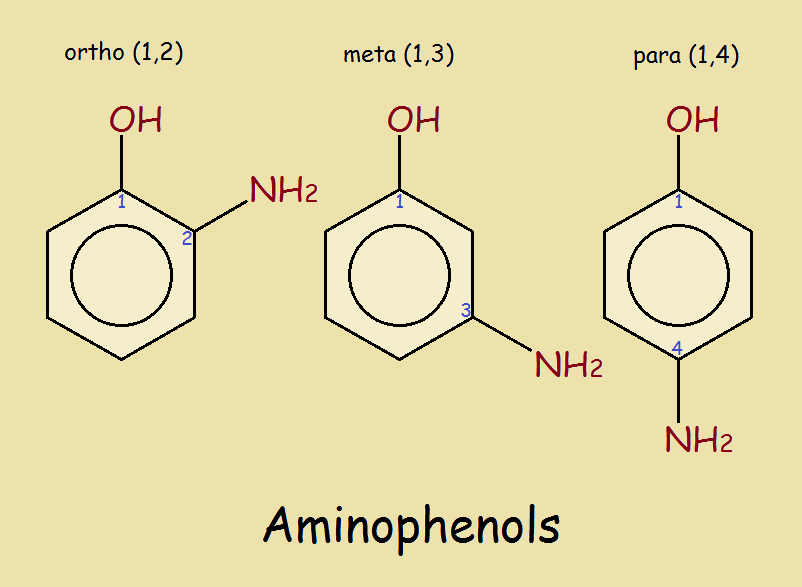
Phenol is not C6H5–, but C6H5OH. It is a compound. Now there are five hydrogen atoms directly attached to the ring. If we choose to replace one, we need to know which one it was! This is where the “para” part comes in. Since the hydroxyl group is attached to carbon atom #1 and the nitrogen is attached to carbon atom #4, it is para-aminophenol.
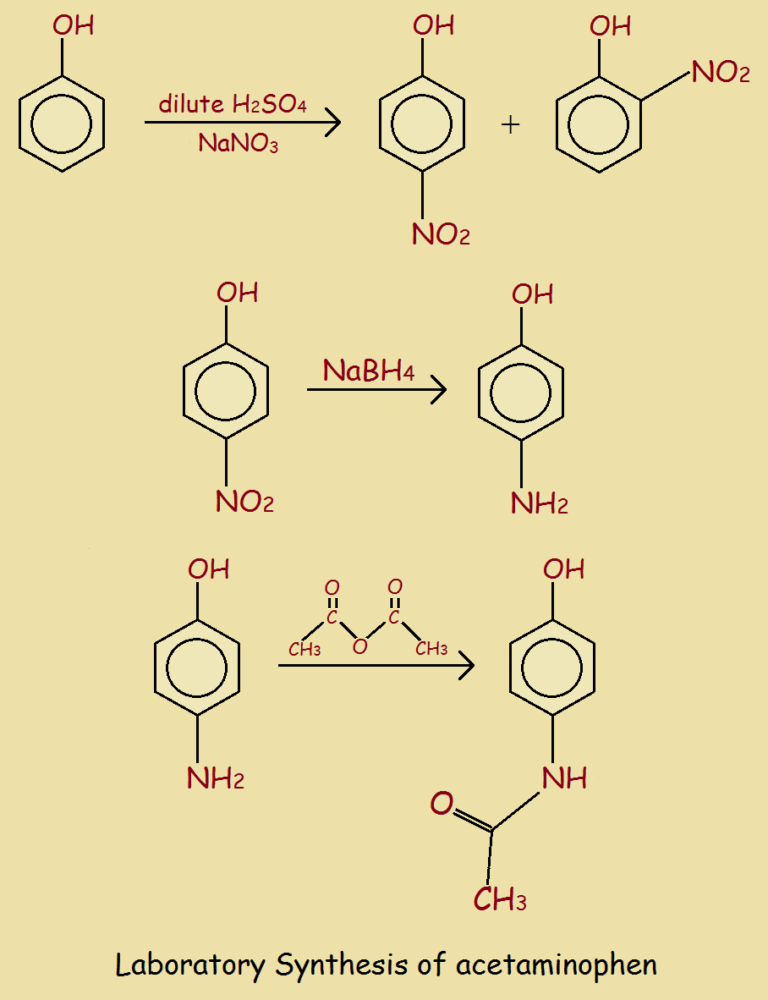
How Is Tylenol Made in the Lab?
Just as there is more than one way to travel from Philadelphia to New York City, there are multiple ways to produce acetaminophen in the laboratory. We provide one way.
Although three steps are drawn in this reaction sequence, in an actual laboratory, probably only the third step would be carried out, since para-aminophenol is relatively cheap and easy to purchase commercially.
Its Mode of Action
It is beyond the scope of this article to discuss acetaminophen’s mode of action; it appears to be quite complex. In addition, there is no certainty the mechanisms under consideration will prove to be the correct ones.
What may be worthy of note is that correctly chosen combinations of pain medications that include Tylenol (consult a physician) may be more successful at relieving pain than either medication would be, taken alone.
¹ Yet another frequently used name among clinicians is paracetamol.
Note: You might also enjoy The History of Aspirin
References:
← Back to Food and Health
← Home

I never looked at Tylenol from the chemistry of it. I take a Tylenol for headaches and it seems to do a pretty good job of relieving them.
I agree, Anthony. The thing is this: don’t take what you don’t have to take. We all can endure some pain. But how much medicine can we take unscathed?
I would use acetic anhydride in the final step. I do not know what Me-O-O-O-Me is, which is drawn up in synthesis…
Thanks for catching the missing C’s. Adjusting… -Vince.