 Many with even a casual chemistry acquaintance have heard of such compounds as hydrocarbons, alcohols, ethers, glycols, and so forth. But the majority have probably never heard of oximes. What are oximes? How are they formed? Are they useful?
Many with even a casual chemistry acquaintance have heard of such compounds as hydrocarbons, alcohols, ethers, glycols, and so forth. But the majority have probably never heard of oximes. What are oximes? How are they formed? Are they useful?
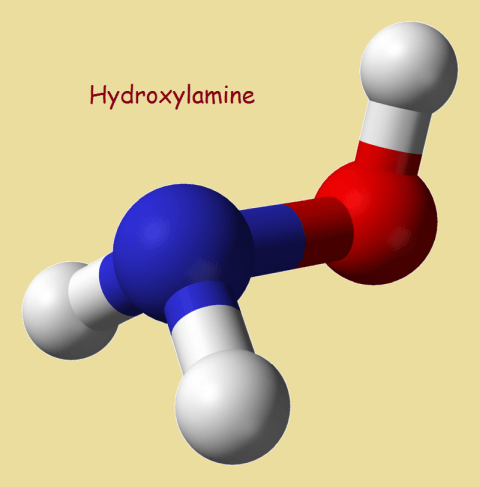
Hydroxylamine
Ammonia’s chemical formula is NH3. Structurally, it resembles a tripod. If one of its hydrogen atoms is replaced by an hydroxyl group, –OH, the result is hydroxylamine, NH2OH.
Two Oxime Varieties
Hydroxylamine combines with aldehydes1 or ketones2 to form aldoximes or ketoximes, respectively. These are two subclasses of oxime.
For example, hydroxylamine combines with acetaldehyde…
CH3–CHO + NH2–OH → CH3–C(H)=N–OH
And hydroxylamine combines with dimethylketone…
(CH3)2=O + NH2–OH → (CH3)2–C=N–OH
Of course there are other synthetic pathways that can be employed to produce oximes.
So the question becomes, Do oximes serve any useful purpose?
Are Oximes Useful?
Different oximes offer a range of applications. One famous oxime, dimethylglyoxime, or DMGH2, was used historically in the analysis of nickel. The scheme for this can be seen in the image below.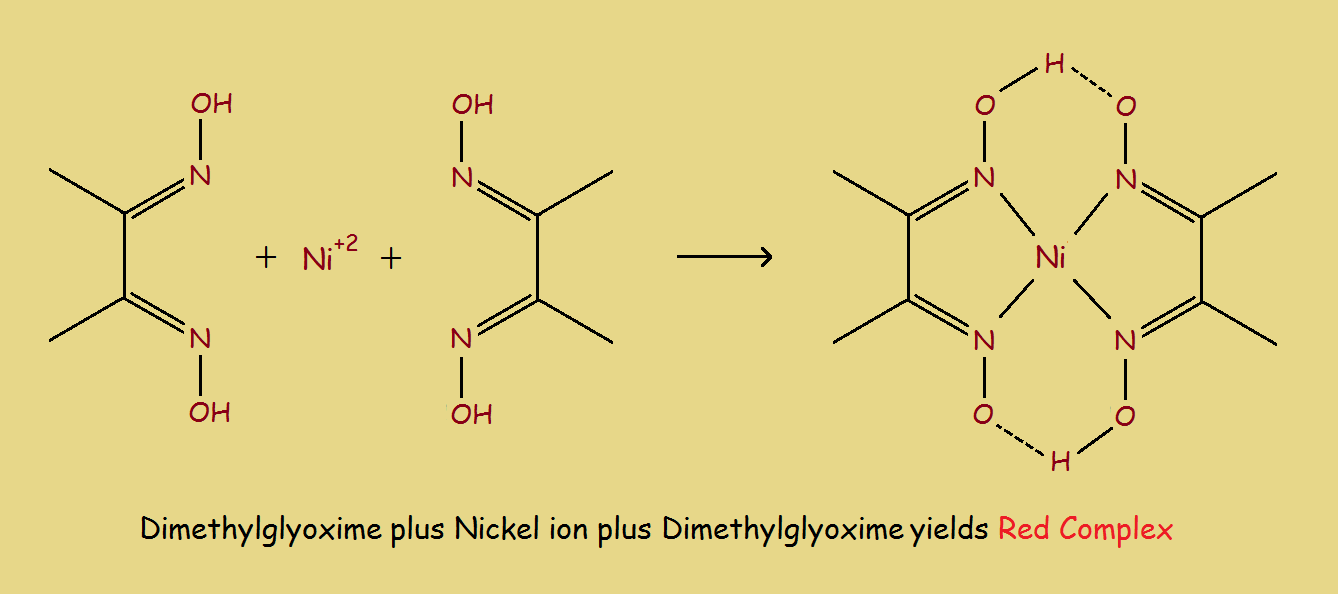
However, there is one use of oximes, an intermediate use, that most of us are very familiar with, especially from approximately WW2 on. What is that? The manufacture of Nylon. One form of the polyamide polymer was developed in the late 1940s, Nylon 6.
There are a number of steps leading to the production of Nylon 6, most of them, with mechanistic detail are discussed in the PSLC reference below. We skip some of the detail for clarity. Note the related images.
Initial Steps to Nylon 6
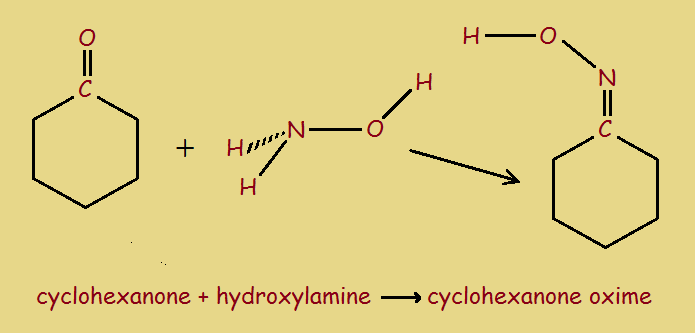
1. Combine the ketone cyclohexanone with hydroxylamine to form cyclohexanone oxime.
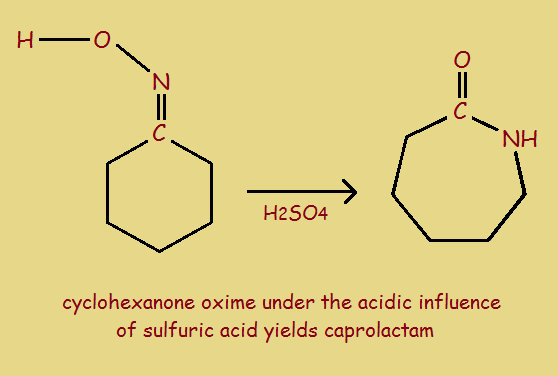
2. Acidify the oxime, which initiates a Beckmann rearrangement. The reaction product is then neutralized with ammonia, to yield the 7-member ring compound caprolactam.
3. Heat the lactam in a nitrogen atmosphere to approximately 533° Kelvin. This opens the ring and initiates polymerization to form Nylon 6.
In Conclusion
There are a number of other specific uses for certain oximes, however we will not discuss those here. We do note that oximes are beneficial to the organic chemist, in purifying carbonyl compounds.
Sometimes oximes are used in multistep chemical synthesis to protect a carbonyl compound so that another synthetic step that would damage the carbonyl can be accomplished. Following that step, the carbonyl can be regenerated.
Yes, the formation of an oxime is generally reversible. This greatly adds to its utility.
1 Compounds with the generic structure R-C(O)H, often written R-CHO, where R is some form of hydrocarbon or hydrocarbon derivative.
2 Compounds, similar to aldehydes, except the H atom is replaced by another hydrocarbon derivative, R’. Hence, R-CO-R’. R and R’ may or may not be the same.
Extra Credit Video: We include the following short video explaining the specific Beckmann rearrangement we have discussed. It is somewhat detailed, and may primarily interested students of organic chemistry. Enjoy…
Note: You might also enjoy Organic Chemistry: What is a Lactam?
References:
← Back to Classic Science
← Home

I have never heard of oximes before but I saw a video of nylon being produced at the interface between two liquids. Very interesting.