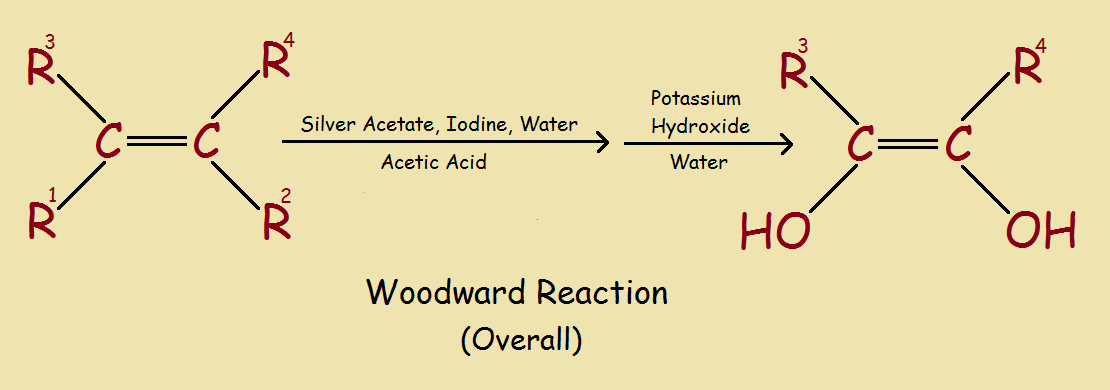
 Silver acetate, in combination with iodine, forms the initial reactant package for the Woodward reaction. This reaction, carried to completion, selectively converts an alkene into a cis-diol.
Silver acetate, in combination with iodine, forms the initial reactant package for the Woodward reaction. This reaction, carried to completion, selectively converts an alkene into a cis-diol.
The prefix cis- refers to the addition of two atoms (or groups of atoms) to the same side of a molecular double bond. Trans-, when used, refers to addition across the double bond – of one atom or group to one side, one to the other side.
The Mechanism
The mechanism is illustrated in the image (below) up to the point of hydrolysis. The product of that hydrolysis is pictured in the introductory image.
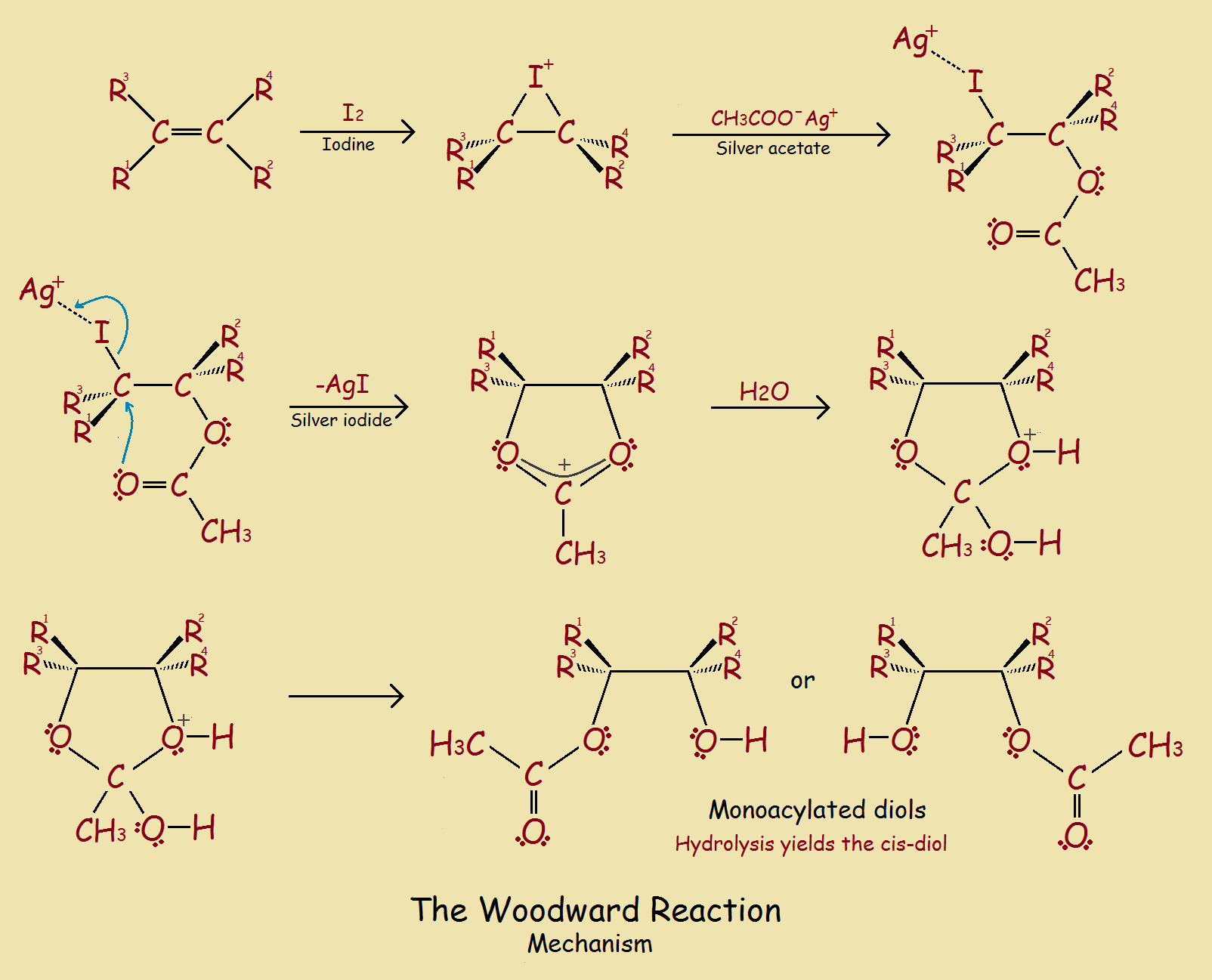
We see, first, the iodine splits, the I atom adding to the double bond. In the next part of the reaction, the silver atom attaches to the iodine, and the acetate group adds to the second carbon atom. Silver iodide then forms, leaving the species.
The result is the formation of the heterocycle. When water is added, the ring and its appendages undergo changes that result in one of two monoacylated diols. Hydrolysis removes the acylation, giving the diol.
Usefulness
If a mixture of isomers, both cis- and trans- sufficed, the Woodward reaction might not be considered all that important. But where specificity is important, yields of the desired isomer would be cut, and costs would rise. The Woodward reaction is of particular importance in steroid chemistry.
Note: You might also enjoy Organic Chemistry: Pushing Electrons
References:
- ACS Publications: cis-Hydroxylation of a Synthetic Steroid Intermediate with Iodine, Silver Acetate and Wet Acetic Acid by R. B. Woodward and F. V. Brutcher Jr.
- Science Direct: The stereochemistry of woodward cis-hydroxylation in some steroidal olefins
