 Clearly cyclobutadiene is not aromatic. Is cyclobutadiene antiaromatic? Or is its lack of stability due to other factors?
Clearly cyclobutadiene is not aromatic. Is cyclobutadiene antiaromatic? Or is its lack of stability due to other factors?
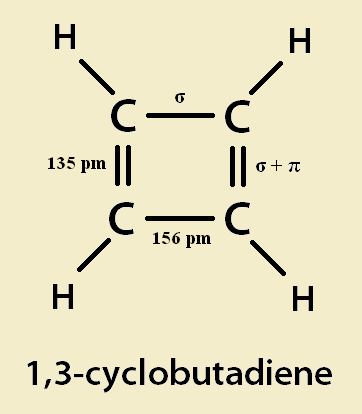
Cyclobutadiene is a four-carbon hydrocarbon. The –diene part of its name indicates it has two double bonds. The cyclo– part indicates it is not a chain structure, but a cyclic (ring) structure. Now double bonds hold atoms tighter than single ones do. So they are shorter than single bonds. This causes cyclobutadiene to assume a rectangular shape. If it were square instead, it would indicate the four carbon to carbon bonds were identical, and aromatic. Cyclobutadiene’s four hydrogen atoms point away from the ring.
Analyzing the Structure
Cyclobutadiene is flat. The two double bonds of cyclobutadiene are conjugated. That is, their ring structure alternates double bond, single bond, double bond, single bond. In addition, there is no break in this conjugation. It is closed.
This contrasts with 1,3-cyclopentadiene, which has a double bond, single bond, double bond, single bond, single bond, structure, featuring two single bonds next to each other. Such a structure is not completely conjugated.
Since a flat, conjugated, ring structure could support a ring current, cyclobutadiene might seem a likely candidate for aromaticity, whereby all bonds of the closed ring assume equal length, stabilizing the structure. This does not happen for cyclobutadiene. Why not?
Cyclobutadiene Antiaromatic? Other Factors?
First off, cyclobutadiene’s double bonds are shorter than its single bonds. This would not be the case if cyclobutadiene was aromatic. Besides, Hückel’s rule states that a conjugated hydrocarbon is aromatic if its ring has 4n+2 π-electrons. But cyclobutadiene has 4 π-electrons. It is antiaromatic. It is unstable rather than stabilized. In fact, cyclobutadiene is highly reactive.
Is cyclobutadiene so unstable primarily because of its antiaromaticity? There are other factors that may well play a greater role. The journal Chemical Communications, Issue 67 of 2012, includes the article “Is cyclobutadiene really highly destabilized by aromaticity?” accredited to Judy I-Chia Wu, et. al., that cites a number of other factors, including “angle strain, torsional strain, and Pauli repulsion between the parallel CC bonds.” Let us briefly consider each of these.
Angle Strain
The ideal sp² bond-angle between two carbon atoms is 120º. But the bond-angles in cyclobutadiene are just 90. This destabilizes cyclobutadiene considerably. In fact, it is quite probably the main factor.
Torsional Strain
When bonds are too close, they tend to twist or contort to relieve the strain. This form of strain adds to the angle strain previously mentioned. Could there also be a destabilizing factor something like the banana bond (bent bond) phenomenon of the cyclopropyl ring?
Pauli Repulsion
As bonding electrons from different bonds approach each other, Pauli exclusion principle repulsion can force electrons into higher energy states, contributing to destabilization.
In Conclusion
It might be suggested destabilization of cyclobutadiene needn’t be attributed to either this factor or that factor, but to the total contribution of them all. Is cyclobutadiene antiaromatic? Whatever the case, cyclobutadiene certainly does not meet the Hückel criteria for aromaticity. Curiously, though, its dianion does exhibit aromaticity.
You may find this YouTube video Introduction to Aromaticity and Antiaromaticity helpful.
Relevant additional articles by the author:
- The Aromatic Cyclopentadienyl Anion
- Huckel’s Smallest: Aromatic Cyclopenyl Cation
- Is Cyclopropenone Aromatic or Not?
References:
- RSC: Is cyclobutadiene really highly destabilized by antiaromaticity?
- Rutgers University: Aromatic Compounds
- Georgia State University Hyper-Physics: Pauli-Repulsion in Ionic Molecules
- ACS: Aromaticity of the Cyclobutadiene Dianion: Structural Characteristics and Magnetic Properties
← Back to Classic Science
← Home

This post is very simple to read and without leaving any details out. Great work!